Our Master Files solution for Trial Master Files is now online. Now you can easily link documents to your Trial Master File within seconds. Each new document version is automatically synchronised with the trial master file. The result: You are … Read More
Docuply
The quality management system for pharmaceutical SMEs and start-ups. Pre-validated, ready to use and securely hosted in the EU cloud. The result: No more hassle with audits and inspections.

As a tailor-made quality management system for small and medium-sized companies, Docuply offers the ideal solution for the following types of companies:
Contract Research Organisations
Docuply optimises quality management for Contract Research Organisations (CRO) and ensures audit readiness. The TMF feature (CDISC reference model) gives sponsors insight into study progress at any time, while SOPs for third parties can be easily managed.
Biotech Start-Ups
Docuply offers biotech start-ups a ready-to-use, pre-validated quality management system with attractive pricing. The intuitive user interface does not require lengthy training, and the Training Hub simplifies the onboarding of new employees – which is ideal for ensuring rapid growth.
Pharmaceutical Service Providers
With Docuply, pharmaceutical service providers such as cleanroom cleaning and logistics service providers optimise their training management. The Training Hub ensures efficient training, and the training matrix ensures that there are no gaps in the documentation of training records.
Our mission
With Docuply, we enable pharmaceutical and biotech companies to ensure smooth and secure quality management with third parties and distributed teams. This allows you to avoid chaotic audits and downtime during an inspection. Ultimately, this leaves more time for what really matters: Making the world a little bit better with new medicines.

Digitise and automate your daily QM tasks with Docuply. On average, our users save 32% of their daily time and additionally halve the findings during audits.
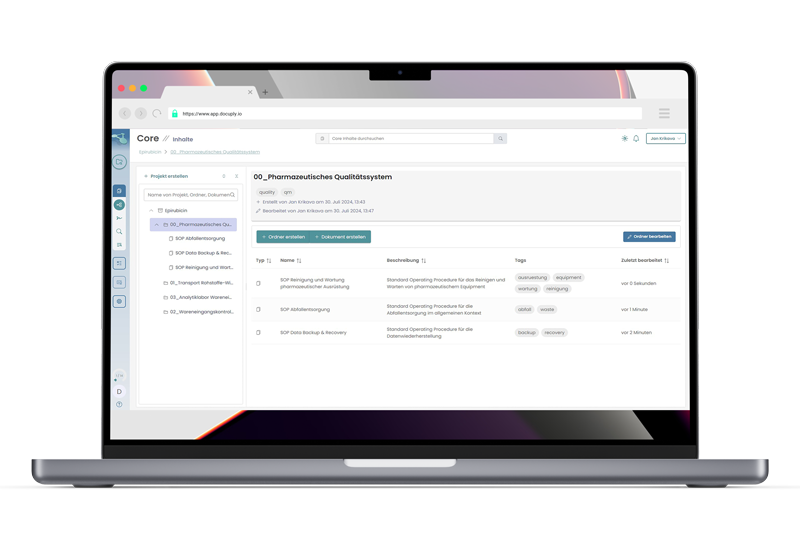
Compliant document management, lightning fast through cloud technology.
All features at a glance
- Setup and onboarding in ten minutes
- Pre-validated system (IQ & OQ) for immediate use
- EU Cloud with 99.95+% availability
- ISO27001 certified and GDPR-compliant
- GLP-, GCP- and GMP-compliant document management
- Digital signature compliant with CFR 21 Part 11 and Annex 11
- Private blockchain audit trail
- Straightforward inviting of third parties and external users
- Modern encryption for all uploaded documents
- Support for all common document types
- Text recognition (OCR) for most document types
- Trial master files with CDISC reference model
- Centralised management of SOP training and other training courses
- Find your documents easily with the help of tags
- API for seamless integration into your IT landscape (in the near future)
- Find more future features on our product roadmap

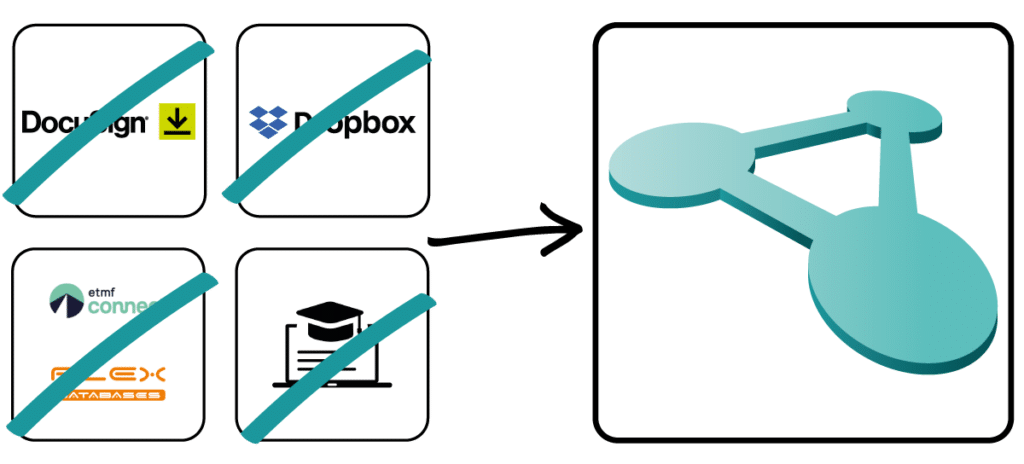
Don't rely on stand-alone solutions for your quality management and use a fully integrated solution with Docuply without the hassle of jumping back and forth between different applications.
What experts say about us
"Many companies are struggling with isolated IT solutions and the big challenge of how to apply or adapt established IT systems in the necessary interaction with more and more external business partners to meet regulatory requirements at the same time. For this, the young startup Docuply offers a very simple and at the same time very flexible solution that is ready to use thanks to its cloud architecture."
Known from


















Register now and test Docuply free of charge
During the test period, you can cancel at any time without obligation. There are no costs.

News
Here you can find news about Docuply.

Master Files – Autopilot for Trial Master Files, Drug Master Files and all other Master Files
After plenty of feedback from our customers and other interested users, we will develop the Master Files solution module in the coming weeks. Using the Master Files solution, you can link documents from Document Management to the desired Master File, … Read More
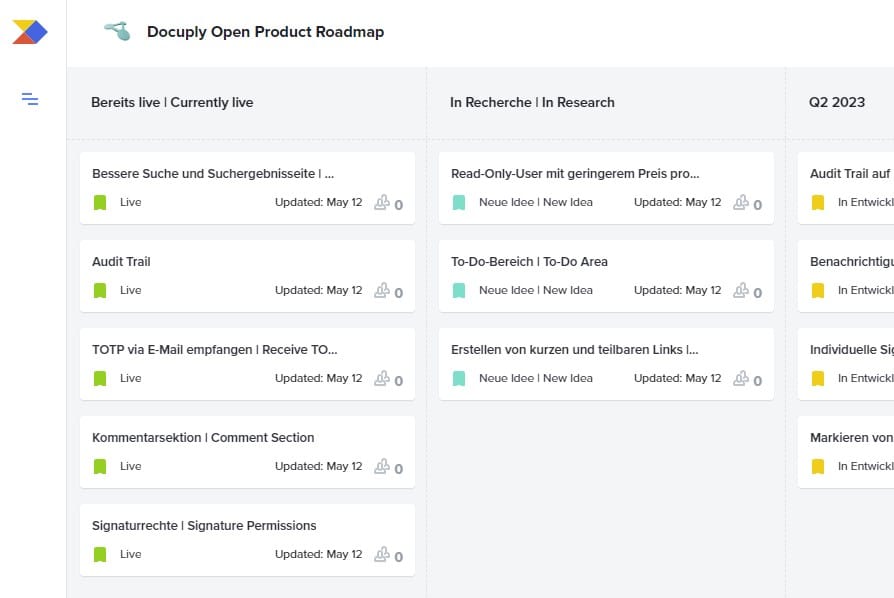
Our new Open Product Roadmap is available
Our new Open Product Roadmap is available and replaces our old Open Product Roadmap. The new Product Roadmap offers the advantage to rate single feature proposals. To do this, you have to click on a feature map and then rate … Read More
Contact
Do you have any questions or suggestions? Please do not hesitate to contact us.

