Understanding the Significance of FDA 21 CFR Part 11 in the Pharma Industry

- Electronic Records: Part 11 mandates that electronic records be secure, reliable, and accurate throughout their lifecycle. This includes requirements for electronic documentation of manufacturing processes, batch records, laboratory results, and other critical data.
- Electronic Signatures: Part 11 permits the use of electronic signatures as legally binding alternatives to traditional handwritten signatures. It sets guidelines for the use of cryptographic controls to ensure the authenticity and non-repudiation of electronic signatures.
- Audit Trails: Part 11 requires systems to have audit trails that capture a chronological record of all system activities, including user actions, data modifications, and system events. These audit trails aid in traceability, accountability, and data integrity verification. Docuply as a document management system for the pharmaceutical industry offers an audit trail by default.
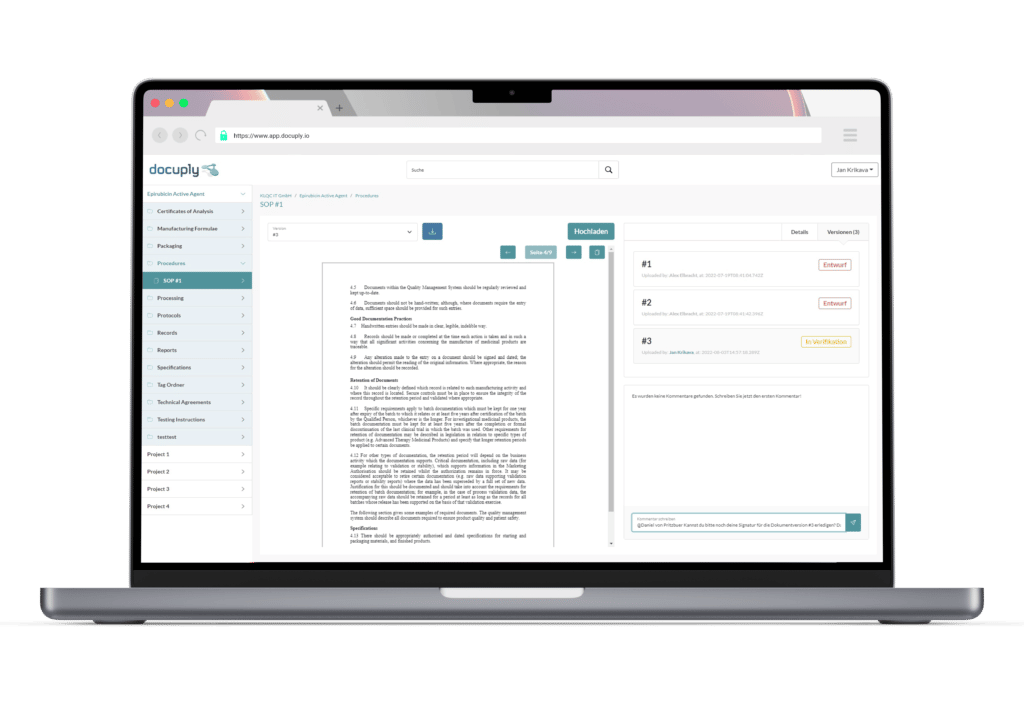
With Docuply, you and your third parties can move smoothly and GLP-compliant from preclinical studies to clinical trials.
Challenges in Implementation
Implementing FDA 21 CFR Part 11 can pose challenges for pharmaceutical companies. Organizations must invest in robust electronic systems and software solutions that meet the stringent requirements outlined by the regulation. Additionally, training employees on proper system usage, data security, and electronic signature protocols is essential. Maintaining compliance in a rapidly advancing technological landscape also necessitates regular updates, system validations, and ongoing monitoring.
Conclusion
FDA 21 CFR Part 11 plays a vital role in ensuring data integrity, security, and compliance in the pharmaceutical industry. By adhering to the regulation’s requirements for electronic records, signatures, and audit trails, pharmaceutical companies can protect patient safety, enhance operational efficiency, and maintain regulatory compliance in an increasingly digital era.
Test all the advantages of a digital document management system specially developed for the pharmaceutical and biotech industry now without obligation. You can get started in just five minutes, see for yourself.
Quality management in the pharmaceutical and biotech industry: Current challenges in the cooperation between pharmaceutical companies and CROs, CMOs and CDMOs
"The increasing decentralization of the supply chain in the pharmaceutical industry poses major challenges for companies. In a regulated environment, regulatory requirements must be implemented in a compliant manner while adhering to "Good Documentation Practice" with business partners in a global environment. The global networking and faster availability of information in large numbers are pushing our existing document management systems to their limits in terms of timely processing in compliance with GMP requirements. Therefore, we need to rethink our current way of working to enable effective and efficient document management with new IT solutions."
