A Comprehensive Guide to Crafting a Successful Marketing Authorization Application

When it comes to bringing a new pharmaceutical product to market, one crucial step is the submission of a Marketing Authorization Application (MAA). Also known as a New Drug Application (NDA), this detailed submission is the gateway to obtaining regulatory approval from agencies like the FDA in the United States or the EMA in Europe. In this article, we’ll delve into the essential components of an MAA and how to navigate this intricate process.
Administrative Details: Setting the Foundation
The journey begins with providing accurate administrative information. Include contact details for the applicant, co-applicants, or partners. A well-crafted cover letter and a comprehensive table of contents are your first opportunities to make a positive impression on regulatory authorities.
Summary: Capturing the Essence
Create a succinct yet compelling summary of the application. Highlight the product’s benefits, potential risks, and the intended use or indication. A clear and concise summary sets the tone for the rest of the application.
Quality Documentation: Ensuring Excellence
Regulators emphasize the quality and consistency of the product. Detail the formulation, manufacturing processes, and strict quality controls. Specifications for raw materials, finished products, and packaging materials, along with stability data, illustrate the product’s durability and quality over time.
Preclinical Data: Safety First
Robust preclinical data is pivotal for demonstrating product safety. Furnish results from laboratory studies and animal testing, shedding light on potential risks. Cover pharmacology, toxicology, and ADME (absorption, distribution, metabolism, excretion) characteristics comprehensively.
Clinical Data: Proof of Efficacy and Safety
Clinical trials are the cornerstone of any MAA. Present detailed outcomes from these trials that highlight the product’s safety and efficacy. Include study protocols, investigator brochures, and in-depth clinical trial reports, along with patient demographics and statistical analyses.
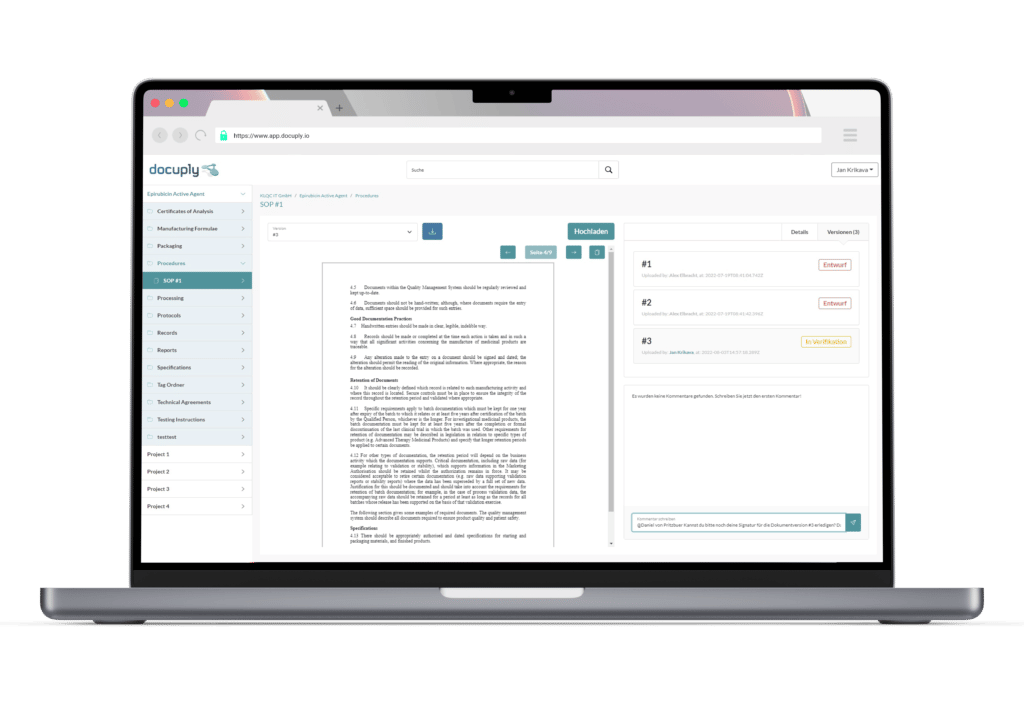
The data basis for a marketing authorisation application requires structured document management as part of quality management. Find out now how Docuply can help you with this.
Pharmacovigilance and Risk Management: Prioritizing Safety
Address strategies for monitoring the product’s safety post-launch. Articulate plans to manage and communicate potential risks effectively, showing your commitment to patient safety throughout the product’s lifecycle.
Regulatory Strategy: Navigating the Maze
Your regulatory strategy and justification play a crucial role. Explain how your product aligns with safety, efficacy, and quality standards. Provide a solid scientific rationale for the proposed indication and dosing.
Labeling and Packaging: The Face of Your Product
Devote attention to proposed labeling, encompassing indications, warnings, precautions, and instructions for use. Design packaging materials that are both functional and visually appealing.
Environmental Risk Assessment: A Responsible Approach
If applicable, address potential environmental impacts. Highlight your commitment to environmentally conscious practices and underscore your product’s sustainability.
User Fees and Payments: Financial Clarity
Transparently present details of the fees and payments associated with your application. Demonstrating fiscal responsibility enhances your credibility with regulatory bodies.
Conclusion
Navigating the process of crafting a Marketing Authorization Application may be intricate, but a meticulous and well-prepared submission significantly increases your chances of regulatory approval. By meticulously addressing each component, from administrative details to environmental considerations, you set the stage for a successful journey toward bringing your pharmaceutical product to market. Collaborating with regulatory experts can further enhance your application’s strength and adherence to specific agency requirements.
Test all the advantages of a digital document management system specially developed for the pharmaceutical and biotech industry now without obligation. You can get started in just five minutes, convince yourself.
Quality management in the pharmaceutical and biotech industry: Current challenges in the cooperation between pharmaceutical companies and CROs, CMOs and CDMOs
"The global networking and faster availability of information in large numbers are pushing our existing document management systems to their limits in terms of timely processing in compliance with GMP requirements. Therefore, we need to rethink our current way of working to enable effective and efficient document management with new IT solutions."
