Sharepoint as a document management system in the pharmaceutical and biotech industry?

SharePoint is a popular document management system, but it may not be the best choice for the pharmaceutical and biotech industries. There are several reasons why SharePoint is not suitable for these industries, including lack of regulatory compliance and difficulty managing large amounts of data. A major problem with SharePoint is that it does not comply with the regulatory standards that apply to the pharmaceutical and biotech industries. These industries are highly regulated and must adhere to strict guidelines set by the FDA and other regulatory agencies. SharePoint does not have built-in compliance capabilities and may not be able to meet the specific requirements of these industries. For example, the FDA has Electronic Records and Electronic Signatures (ERES) guidelines that set standards for the validation, security, and integrity of electronic records and signatures. SharePoint may not be able to meet all the requirements of these guidelines, putting it at risk of non-compliance.
Another limitation of SharePoint is the difficulty in managing large volumes of data. The pharmaceutical and biotechnology industries often handle large amounts of data, including research results, clinical trial data and patient information. SharePoint’s data management limitations can make it difficult for these industries to effectively organize and manage their data. For example, SharePoint does not provide built-in support for version control, which is essential for managing data in the pharmaceutical and biotech industries. This can lead to confusion and errors when multiple versions of a document exist and it is not clear which version is the most current and correct. In addition, SharePoint’s search capabilities are limited, making it difficult to find specific documents or data in the system. This can be a major problem in the pharmaceutical and biotech industries, where large amounts of data are often generated and quick and easy access to specific information is required.
Pharmaceutical and biotech companies often have multiple teams working on a project, and they need a system that allows them to easily share documents and collaborate. SharePoint’s collaboration features may not be as advanced as other systems, which can make it difficult for teams to collaborate effectively. In summary, while SharePoint is a popular document management system, it may not be the best choice for the pharmaceutical and biotech industries due to the lack of regulatory compliance and the difficulty in managing large amounts of data. These industries need a system that is specifically designed to meet their unique needs and comply with strict regulatory requirements. The system also needs to offer better data management, search capabilities, and collaboration features.
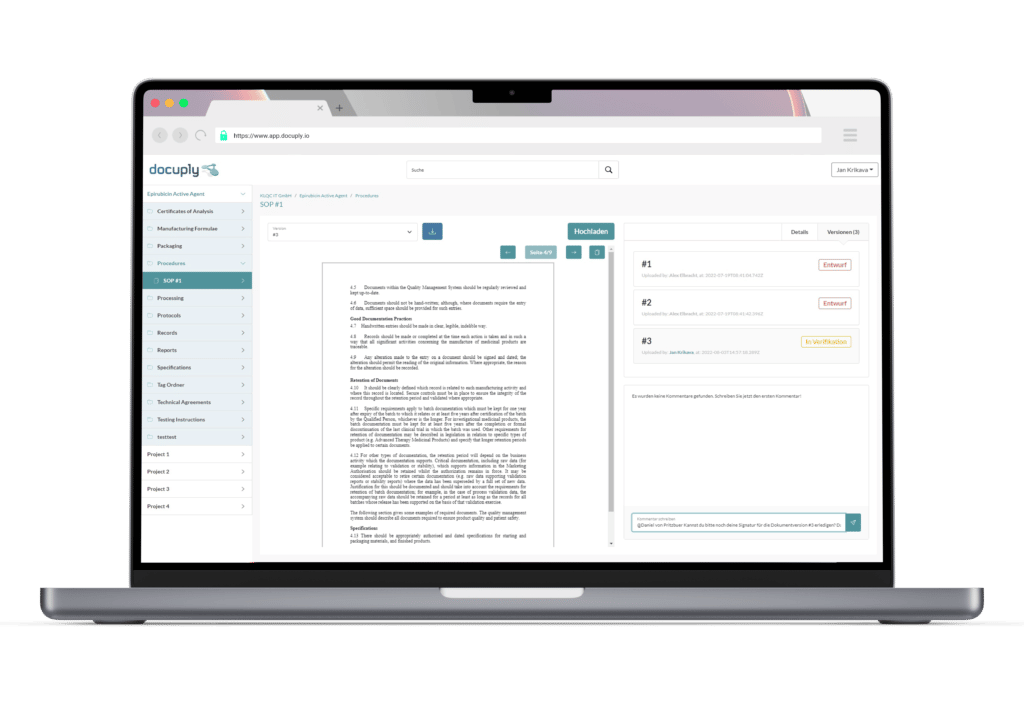
A document management system tailored specifically to pharmaceutical companies: Docuply meets regulatory requirements and offers digital signing of your documents with a single solution.
In addition, SharePoint’s search capabilities are limited, making it difficult to find specific documents or data in the system. This can be a major problem in the pharmaceutical and biotech industries, where large amounts of data are often generated and quick and easy access to specific information is required. Pharmaceutical and biotech companies often have multiple teams working on a project, and they need a system that allows them to easily share documents and collaborate. SharePoint’s collaboration features may not be as advanced as other systems, which can make it difficult for teams to collaborate effectively.
In summary, while SharePoint is a popular document management system, it may not be the best choice for the pharmaceutical and biotech industries due to the lack of regulatory compliance and the difficulty in managing large amounts of data. These industries need a system that is specifically designed to meet their unique needs and comply with strict regulatory requirements. The system also needs to offer better data management, search capabilities, and collaboration features.
Test all the advantages of a digital document management system designed specifically for the pharmaceutical and biotech industry, without any obligation. You can get started in just five minutes, convince yourself.
Quality Management in the Pharmaceutical and Biotech Industry: Current Challenges in the Collaboration between Pharmaceutical Companies and CROs, CMOs and CDMOs
"The increasing decentralization of the supply chain in the pharmaceutical industry poses major challenges for companies. In a regulated environment, regulatory requirements must be implemented in a compliant manner while adhering to "Good Documentation Practice" with business partners in a global environment. The global networking and faster availability of information in large numbers are pushing our existing document management systems to their limits in terms of timely processing in compliance with GMP requirements. Therefore, we need to rethink our current way of working to enable effective and efficient document management with new IT solutions."
