Streamlining Document Approval Workflows with Custom Workflows in Pharma Document Management Systems

Introduction
In the pharmaceutical industry, efficient document management is crucial for maintaining compliance, ensuring data integrity, and expediting time-sensitive processes. Custom workflows within pharma document management systems play a pivotal role in streamlining document approval processes. By tailoring workflows to specific requirements, pharmaceutical companies can improve efficiency, reduce errors, and enhance collaboration. This article explores the benefits of custom workflows in streamlining document approval within the pharmaceutical sector.
Eliminating Manual Handoffs and Delays
Traditional document approval processes often involve physical handoffs, multiple stakeholders, and tedious coordination. Custom workflows in pharma document management systems automate these processes, eliminating manual handoffs and reducing delays. With custom workflows, organizations can define sequential or parallel signing tasks, assign roles to individuals, and set deadlines. This automation minimizes the time spent on administrative tasks and ensures smooth and timely document approval, enabling pharmaceutical companies to accelerate their operations.
Enhancing Collaboration and Visibility
Effective collaboration is crucial in the pharmaceutical industry, where cross-functional teams work together on complex projects. Custom workflows provide a centralized platform where stakeholders can collaborate seamlessly, review documents, and provide feedback. These workflows enable real-time notifications and alerts, ensuring that everyone involved stays informed about the progress of the approval process. By promoting transparency and visibility, custom workflows foster effective collaboration, reducing the risk of miscommunication and ensuring that approvals proceed smoothly.
Increasing Accuracy and Reducing Errors
Manual document approval processes are prone to errors, which can have serious consequences in the pharmaceutical industry. Custom workflows enforce standardized approval processes, reducing the likelihood of errors and improving accuracy. By defining clear steps, roles, and responsibilities within the workflow, organizations can ensure that the right individuals review and approve documents. Additionally, custom workflows can incorporate validation rules and data verification, flagging any inconsistencies or errors, thereby improving data integrity and reducing the risk of regulatory non-compliance.
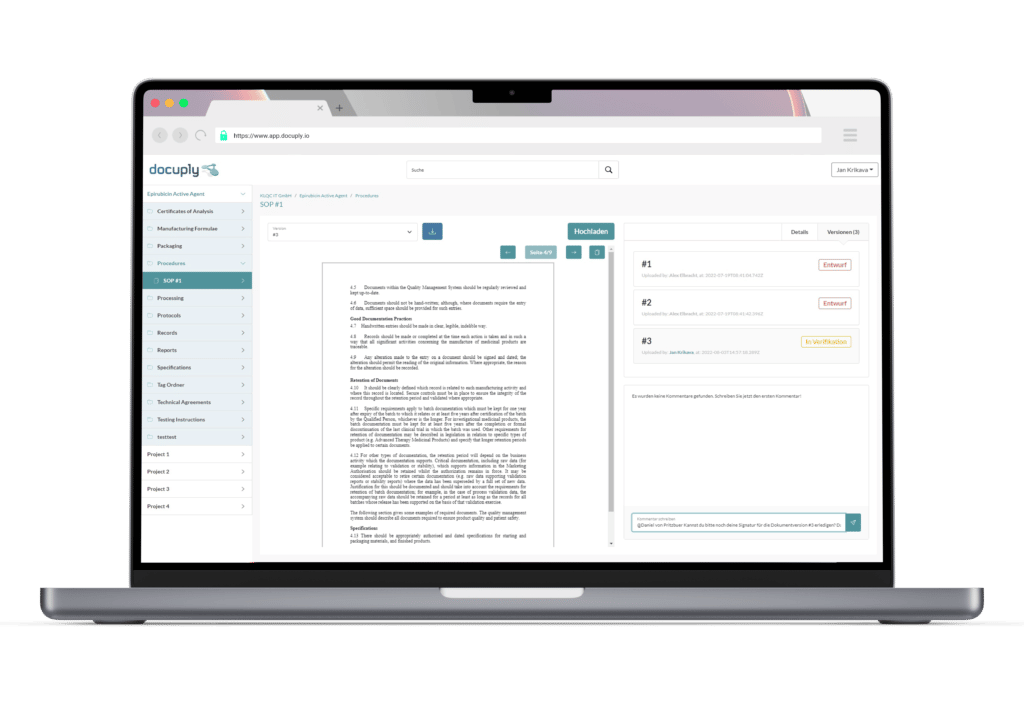
With Docuply, you and your third parties can move smoothly and GLP-compliant from preclinical studies to clinical trials.
Enhancing Regulatory Compliance
Regulatory compliance is a top priority for pharmaceutical companies. Custom workflows in document management systems can be tailored to align with industry regulations, such as FDA 21 CFR Part 11. These workflows enable organizations to implement the necessary controls and documentation requirements, ensuring compliance with regulatory guidelines. Custom workflows also facilitate the capture and retention of audit trails, providing a comprehensive record of the document approval process for regulatory audits. By streamlining compliance processes, custom workflows contribute to risk mitigation and help companies avoid costly penalties.
Improving Efficiency and Productivity
Efficiency and productivity are vital in the fast-paced pharmaceutical industry. Custom workflows optimize document approval processes by automating routine tasks, eliminating bottlenecks, and reducing manual intervention. This automation frees up valuable time and resources, enabling employees to focus on higher-value activities. With streamlined workflows, pharmaceutical companies can improve overall efficiency, reduce operational costs, and achieve faster time-to-market for critical drugs and therapies.
Conclusion
Custom workflows within pharma document management systems offer significant advantages in streamlining document approval processes. By eliminating manual handoffs, enhancing collaboration, reducing errors, ensuring regulatory compliance, and improving overall efficiency, custom workflows empower pharmaceutical companies to navigate the complexities of document management effectively. Investing in a robust document management system that incorporates custom workflows is essential for pharmaceutical organizations seeking to optimize their operations, maintain compliance, and drive innovation in a highly regulated industry.
Test all the advantages of a digital document management system specially developed for the pharmaceutical and biotech industry now without obligation. You can get started in just five minutes, convince yourself.
Quality management in the pharmaceutical and biotech industry: Current challenges in the cooperation between pharmaceutical companies and CROs, CMOs and CDMOs
"The increasing decentralization of the supply chain in the pharmaceutical industry poses major challenges for companies. In a regulated environment, regulatory requirements must be implemented in a compliant manner while adhering to "Good Documentation Practice" with business partners in a global environment. The global networking and faster availability of information in large numbers are pushing our existing document management systems to their limits in terms of timely processing in compliance with GMP requirements. Therefore, we need to rethink our current way of working to enable effective and efficient document management with new IT solutions."
