Docuply
The electronic quality management system for pharmaceutical SMEs and start-ups. Pre-validated, ready to use and with secure hosting in the EU cloud. The result: No more hassle with audits and inspections.

As a tailor-made electronic quality management system (eQMS) for small and medium-sized pharmaceutical companies, Docuply offers the ideal solution for the following types of companies:
Pharmaceutical Start-Ups & Scale-Ups
Docuply offers pharmaceutical start-ups and scale-ups a ready-to-use, pre-validated eQMS at an attractive price. The intuitive user interface requires no lengthy training, and the Training Hub facilitates the onboarding of new employees – ideal for rapid growth.
Contract Research Organisations
Docuply streamlines quality and data management for contract research organisations (CROs) and ensures audit readiness. The TMF feature (CDISC reference model) gives sponsors insight into study progress at any time, while the smooth invitation of site users and 21 CFR Part 11-compliant signatures simplify daily work.
Pharmaceutical Service Providers
Pharmaceutical service providers such as cleanroom cleaning and logistics service providers optimise their training management with Docuply. The Training Hub ensures efficient training courses and the training matrix guarantees complete compliance with documentation requirements. This reduces internal audit costs to a minimum and increases customer satisfaction in the long term.
Our mission
With Docuply, we enable pharmaceutical and biotech companies to ensure smooth and secure quality management with third parties and distributed teams. This allows you to avoid chaotic audits and downtime during an inspection. Ultimately, this leaves more time for what really matters: Making the world a little bit better with new medicines.

Digitise and automate your daily QM tasks with Docuply and benefit from integrated AI functionalities that speed up your tasks by up to 80%.
Compliant document management, lightning fast through cloud technology.
All features at a glance
- Setup and onboarding in ten minutes
- Pre-validated system (IQ & OQ) for immediate use
- EU Cloud with 99.95+% availability
- ISO27001 certified and GDPR-compliant
- GLP-, GCP- and GMP-compliant document management
- Digital signature compliant with 21 CFR Part 11 and Annex 11
- Private blockchain audit trail
- Straightforward inviting of third parties and external users
- Modern encryption for all uploaded documents
- Support for all common document types
- Text recognition (OCR) for most document types
- Trial master files with CDISC reference model
- Centralised management of SOP training and other training courses
- Find your documents easily with the help of tags
- API for seamless integration into your IT landscape (in the near future)
- Find further future features on our product roadmap.

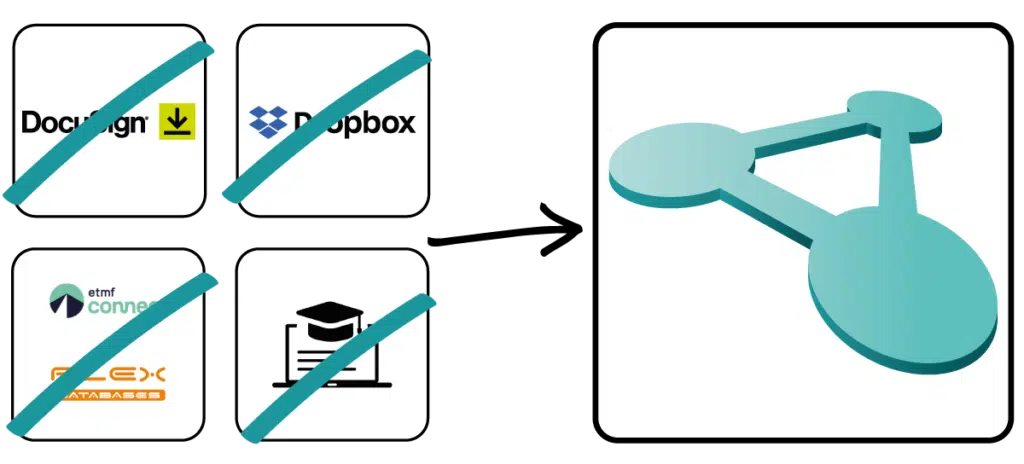
Don't rely on stand-alone solutions for your quality management and use a fully integrated solution with Docuply without the hassle of jumping back and forth between different applications.
What experts say about us
"Docuply takes the stress out of audits and inspections by keeping everything in one place. It simplifies things for pharmaceutical teams and allows them to focus on what matters. Having seen how time-consuming and anxiety-inducing traditional audit preparation can be, I’m excited to support a tool that brings simplicity, transparency and real peace of mind to compliance."
Known from


















Free Document Management for Start-Ups
Up to 8 users, up to 500 MB and 10 electronic signatures (21 CFR Part 11 compliant) per month are free of charge with no strings attached. Avoid SharePoint patchwork and use Docuply, an easily validatable solution.

News
Here you can find news about Docuply.

Master Files – Autopilot for Trial Master Files, Drug Master Files and all other Master Files
Read more »Contact
Do you have any questions or suggestions? Please do not hesitate to contact us.